뇌경색으로 오인된 전신성 홍반성 루푸스 환자의 치명적 수막뇌염
Fatal Meningoencephalitis Initially Misdiagnosed as Cerebral Infarction in a Patient with Systemic Lupus Erythematosus
Article information
Systemic lupus erythematosus (SLE) is a complex systemic autoimmune disorder characterized by a wide range of immunological abnormalities. One of its notable features is the potential to affect the central nervous system (CNS), leading to diverse neurological manifestations including headaches, acute state of confusion, seizure disorder, cerebrovascular disease, and meningoencephalitis. CNS involvement is highly prevalent in SLE patients, ranging from 12% to 75%, and is associated with a poor prognosis. Treatment options such as steroids, azathioprine, rituximab, and intravenous immunoglobulins (IVIGs) have been considered in managing these cases. However, early diagnosis of SLE-related CNS involvement remains challenging due to the rapid progression of symptoms and the need to exclude various alternative diagnoses. In this article, we present a fatal case of an SLE patient with meningoencephalitis, initially resembling an ischemic stroke. Despite administering antibiotics, antiviral agents, IVIGs, steroids, rituximab, and plasma exchange, no clinical improvement was observed.
CASE
A 51-year-old female patient was transferred from another hospital due to sudden disturbed consciousness and right hemiparesis. The patient had a 30-year history of SLE and had been receiving continuous treatment with hydroxychloroquine, prednisolone, and azathioprine. Additionally, she had a previous history of pulmonary tuberculosis and had been diagnosed with a Baker's cyst on her left knee five months prior to admission. During treatment for the Baker's cyst, the patient experienced headache and dizziness, for which she was admitted to another hospital and treated with methylprednisolone. However, a few days later, she developed stupor and right hemiparesis.
MRI of the brain revealed restricted diffusion in the territory of the left middle cerebral artery, along with multifocal mild stenosis in the left middle cerebral artery, anterior communicating artery, and both posterior cerebral arteries (Fig. 1A-D). After transfer to our department, the patient regained alertness but continued to experience dysphagia, dysarthria, persistent right-sided hemiparesis (grade 3-4), and hypoesthesia. Upon admission, the patient was initially treated with antiplatelet agents. However, on the third day, she became confused and developed fever, prompting a cerebrospinal fluid (CSF) study to evaluate encephalitis and vasculitis. The CSF analysis revealed an elevated opening pressure of 220 mmH2O, white blood cell count of 605/mm3 (90% neutrophils), CSF glucose/blood glucose ratio of 15.1/167 mg/dL, protein level of 164 mg/dL (reference range, 15-25 mg/dL), albumin level of 866.3 μg/mL (reference range, 10-30 μg/mL), and adenosine deaminase (ADA) level of 17.6 U/L (reference range, 0-5 U/L). Further laboratory tests, including tuberculosis polymerase chain reaction, alpha-fetoprotein stain and culture, gram stain and bacterial culture, wet smear, and fungus culture from CSF, all returned negative results. Autoimmune antibody tests for various markers such as anti-dipeptidyl-peptidase-like protein 6, anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, anti-leucine-rich glioma-inactivated 1, anti-contactin-associated protein-like 2 receptor, anti-γ-aminobutyric acid type B receptor, anti-N-methyl-D-aspartate receptor, and titin were also negative. Serum complement levels revealed a decrease in C3 (75 mg/dL; reference range: 90-180 mg/dL) and normal C4 (34.2 mg/dL; reference range: 10-40 mg/dL). The erythrocyte sedimentation rate was slightly elevated at 22 mm/hr (reference range: 0-20 mm/hr), while the C-reactive protein level was high at 1.24 mg/dL (reference range: 0-0.5 mg/dL). The anti-double stranded DNA antibody titer was negative, and there were no significant differences compared to previous results.
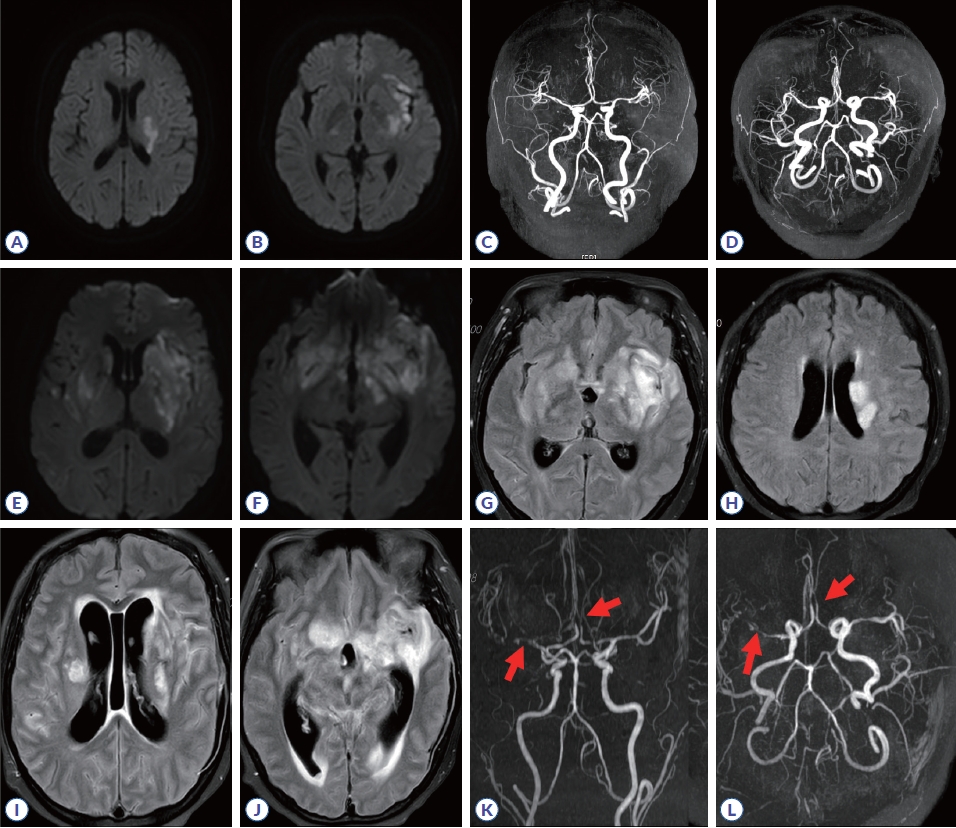
Brain MRI. MR images from the day of admission showing left middle cerebral artery territory infarction (A, B) and multifocal mild stenosis in the left middle cerebral artery, anterior communicating artery, and both posterior cerebral arteries (C, D). Day 9 MR images showing increased extent of multifocal diffusion restriction (E, F) with fluid attenuated inversion recovery (FLAIR) signal change involving bilateral basal ganglia, corona radiata, medial temporal lobes, frontal bases, body of the corpus callosum, and left frontal lobe and diffuse sulcal FLAIR high signal change at bilateral cerebral hemisphere (G, H). Day 15 MR images showing interval progression of a parenchyma lesion with diffusion restriction and FLAIR hyperintensity involving the bilateral basal ganglia, corona radiata, medial temporal lobes, frontal bases, body of the corpus callosum, and left frontal lobe; extension to the thalamus/midbrain/pons and cerebellum (I, J); and a newly developed vascular lesion (arrows) (K, L).
Empirical antibiotic therapy with vancomycin, cefepime, and ampicillin, along with dexamethasone, was initiated on the third day of admission. The patient became more confused the following day, and antiviral therapy with acyclovir was initiated. The disease progression did not slow, and the patient became drowsy with high fever. A follow-up MRI revealed aggravation of the lesion sites (Fig. 1E-H). IVIG therapy with steroid pulse therapy was initiated (0.4 mg/kg for 5 days) on the eighth day; however, no effect was observed, and new-onset arrhythmia and incongruity in breathing and vocal cords developed. Rituximab administration also showed no effect. Considering the ongoing seizures and mental deterioration, plasma exchange was attempted. All treatments proved clinically ineffective; no effects were observed on imaging study (Fig. 1I-L), and the patient died on the 20th day.
DISCUSSION
CNS involvement of SLE patients can occur during any phase of the disease, and symptoms may vary.1 When CNS involvement manifests as acute confusion or is associated with infection, it is often indicative of a poor prognosis.2,3 Here, we present a case of meningoencephalitis initially mimicking acute cerebral infarction, which rapidly progressed and exhibited poor prognostic factors such as an acute state of confusion, ultimately leading to a diagnosis of meningoencephalitis. Although there have been reports of SLE patients with CNS involvement showing improvement after treatment with cyclophosphamide, high-dose steroids, rituximab, IVIGs, or plasmapheresis,4,5 our patient showed minimal improvement with these interventions. Anti-tuberculosis therapy could have been considered as a potential treatment option; however, due to the uncertainty surrounding the diagnosis, lack of risk assessment, and potential side effects of anti-tuberculosis agents, we did not pursue this course of treatment. However, considering the possibility of opportunistic infection or reactivation of latent infection in patients with SLE treated with immune modulators or immunosuppressants6 and a relatively high prevalence of latent tuberculous infection (approximately 10-15%) in patients with SLE,7,8 tuberculous infection was a possible cause of meningoencephalitis. Additionally, the low sensitivity of the tuberculosis polymerase chain reaction test and the CSF findings, including elevated levels of ADA and protein, as well as decreased glucose, further support the potential effectiveness of anti-tuberculous medication.9 Although the final diagnosis remains uncertain because autopsy was not performed, this case highlights the importance of timely diagnosis and prompt initiation of appropriate treatment for CNS involvement in SLE patients. Clinicians should be aware that CNS involvement in SLE patients can present with diverse manifestations, encompassing various etiologies, such as opportunistic infections. Managing CNS involvement in SLE patients can prove challenging and carries a risk of fatality. Further research and clinical studies are warranted to better understand the underlying mechanisms and optimal diagnostic and treatment strategies for CNS involvement in SLE.