Ocrelizumab's Place in the Therapeutic Landscape at its 2019 Launch in France
Article information
Abstract
Background
The treatment landscape for multiple sclerosis (MS) includes a range of disease-modifying therapies (DMTs), from moderate- to high-efficacy agents. Understanding prescribing patterns following the launch of new treatments such as ocrelizumab is essential to assess real-world clinical practices.
Methods
This retrospective cohort study used data from the French National Health Data System between 2019 and 2020. It included 18,912 patients with MS who initiated a DMT during the study period. Patients were categorized according to the initial DMT: moderate-efficacy, or high-efficacy.
Results
Among included patients, 54.5% initiated a moderate-efficacy DMT, 29.5% started ocrelizumab, and 16.0% received a high-efficacy DMT. Most ocrelizumab users (81.2%) were previously treated (non-naïve), compared to 57.6% among moderate-efficacy DMT users and 24.8% among high-efficacy DMT users. Psychiatric and neurological comorbidities were common across all groups (22% overall), with depression reported in 15% of patients.
Conclusion
The strategy of initial high-efficacy treatment was rarely observed in practice during the first year of launch of ocrelizumab in France. Instead, ocrelizumab was predominantly prescribed to previously treated patients who were switching therapies.
INTRODUCTION
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and degenerative disease of the central nervous system, affecting approximately 2.9 million patients worldwide and about 135,000 in France in 2021.1,2 Clinically, MS is categorized into three phenotypic forms of relapsing-remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS), with RRMS and SPMS grouped under the term relapsing-onset MS (relapsing multiple sclerosis [RMS]).
The current therapeutic approach to MS involves disease-modifying therapies (DMTs). Among the high-efficacy DMTs, ocrelizumab, a humanized CD20 monoclonal antibody, has demonstrated a favorable benefit-risk profile in patients with RMS and PPMS.3 This led the European Commission to grant marketing authorization on January 8, 2018.4 In France, ocrelizumab has been reimbursed since February 28, 2019, but only for the treatment of RMS patients with active disease defined by clinical or imaging features.
However, limited data are available regarding the management of MS patients following the introduction of ocrelizumab in France. This study aims to describe the characteristics of patients initiating ocrelizumab compared to those initiating moderate- or other high-efficacy DMTs.
METHODS
Data source and study population
This retrospective, national, population-based cohort study was based on the French national claims database (Système National des Données de Santé [SNDS]). It contains anonymous individual information on sociodemographic characteristics, all non-hospital reimbursed healthcare expenditures (without corresponding medical diagnoses), and all hospital discharge summaries (International Classification of Diseases, 10th revision [ICD-10]-code-based). This claims database currently covers more than 98% of the French population.5
All patients hospitalized (ICD-10 code G35) or covered by a long-term disease (LTD) status for MS between February 28, 2019 and December 31, 2020 and who initiated ocrelizumab or another MS treatment (moderate or high efficacy DMT) were included (Supplementary Table 1). Treatment initiation date (i.e., no dispensation of this medication during the 12 months prior to this date) was defined as the index date. Patients with at least one reimbursement for ocrelizumab under temporary use authorization (ATU)/post-ATU (i.e., before February 28, 2019) and who were previously linked to the PRO-MSactive database were excluded.6 Patients with no reimbursement for MS DMT in the previous 5 years were defined as naïve, while all others were non-naïve.
Study variables and statistical methods
Sex, age, low-income exemption scheme, LTD status, and comorbidities (defined using hospitalization discharge and LTD status) were described. The Charlson comorbidity index has been specifically adapted and validated for use in SNDS studies.7 Descriptive statistics were provided for the overall population, for naïve/non-naïve status and the DMT initiated (moderate, ocrelizumab, or other high efficacy DMT). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
This study was approved by the French Health Data Hub (approval no. 3471595bis from May 6, 2021) and the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés; approval no. 921221 from September 15, 2021).
RESULTS
Among the 142,572 MS patients in the SNDS between January 2013 and December 2020, 63,509 (44.5%) fulfilled the inclusion criterion of at least one dispensation of MS medication of interest between February 2019 and December 2020 (Fig. 1). Finally, 18,912 patients initiated a MS DMT over this period and had sufficient SNDS data history and no loss to follow-up. Ocrelizumab was dispensed to 29.5% of these patients, most of whom were non-naïve (81.2%). Most of the patients who initiated another MS DMT received a moderate-efficacy DMT (77.4%) and were naïve (57.6%). Conversely, three-quarters of patients initiating another high-efficacy DMT were non-naïve.
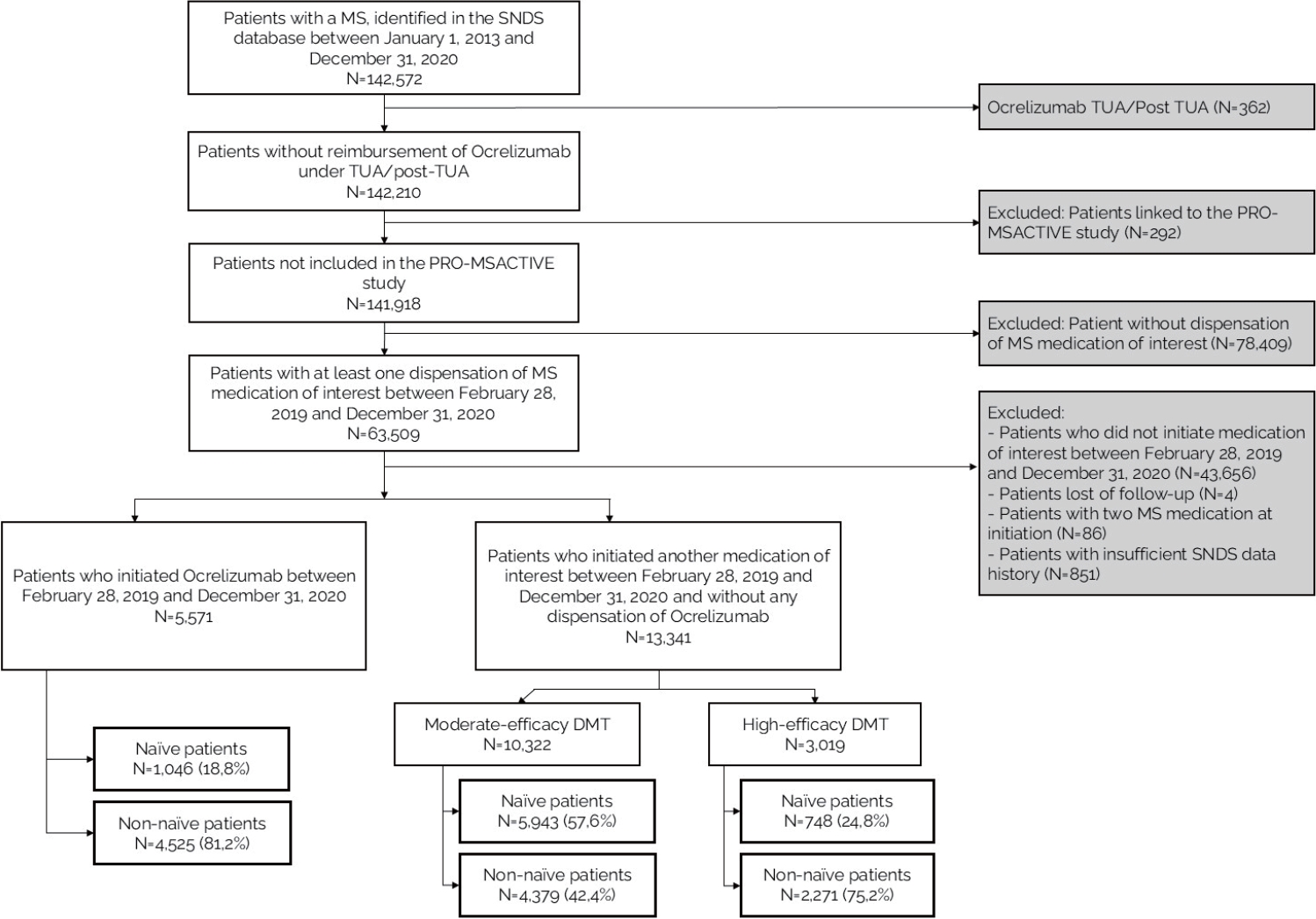
Flowchart of the study. MS, multiple sclerosis; SNDS, Système National des Données de Santé; TUA, temporary use authorization; DMT, disease-modifying therapies.
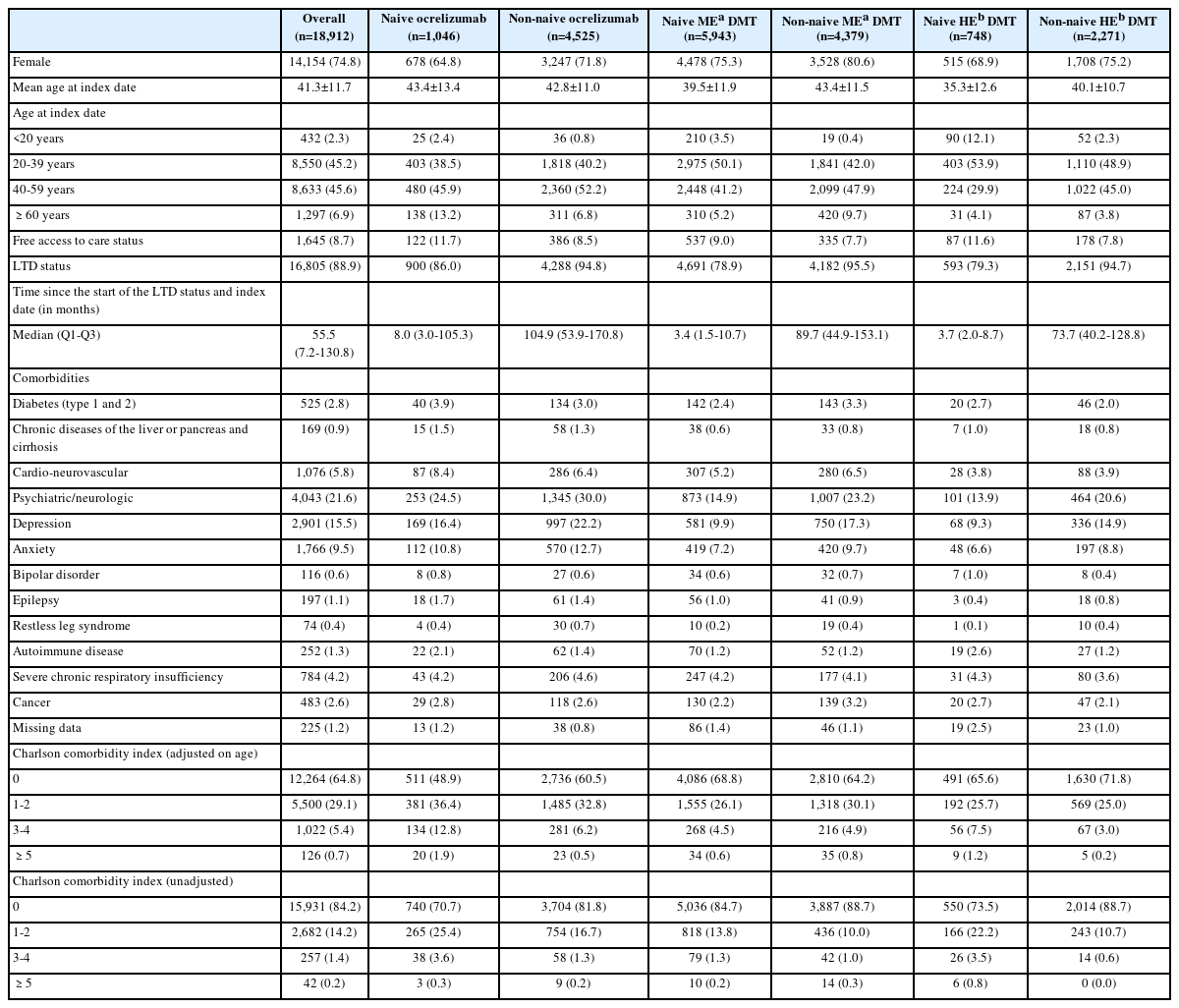
Three-quarters of patients were females and the mean age was 41 years (Table 1). Mean age for ocrelizumab treatment-naïve patients was 43.4 years. About one-third of patients had a Charlson comorbidity index (adjusted for age) of one or more (35.2%). Regardless of subgroup, psychiatric/neurologic comorbidities were the most numerous comorbidity (from 13.9% to 30.0% depending on the subgroup), with 15.5% of the overall population with depression. The prevalence of cardiovascular diseases was slightly higher in naive patients treated with ocrelizumab (8.4%), the most common of which were dyslipidemia (2.5%), and chronic coronary disease (2.2%).
DISCUSSION
This study confirms that the majority of patients treated with ocrelizumab was non-naïve, reflecting the recommendation for its use in active forms of the disease after failure of other DMTs.8 Naïve patients were younger and had a shorter delay between diagnosis and treatment initiation, which suggests earlier intervention in younger, treatment-naïve individuals. This is consistent with the current trend of early therapeutic intervention aimed at slowing disease progression and improving long-term outcomes in MS.9
Regarding comorbidities, psychiatric and neurological disorders were the most common, affecting up to 30% of patients in some subgroups. This is in line with literature showing that MS patients often have higher rates of psychiatric comorbidities such as depression and anxiety, which can impact disease management and overall quality of life.10 The slightly higher prevalence of cardiovascular diseases among naïve patients treated with ocrelizumab also warrants attention. Indeed, comorbidities are crucial factors to consider in therapeutic decision-making.11
Clinicians might have favored ocrelizumab in older treatment-naïve patients due to its infrequent dosing and limited monitoring requirements, which align well with the needs of this population prone to adherence challenges. Additionally, concerns about the safety and tolerability profiles of alternative DMTs-such as progressive multifocal leukoencephalopathy risk with natalizumab, cardiac monitoring with fingolimod, or hepatic adverse events with teriflunomide-might have influenced prescribing patterns in favor of ocrelizumab.
A cross-country comparison highlights the variability in treatment strategies for MS, particularly regarding the use of high-efficacy therapies like ocrelizumab. Real- world studies from Italy, USA, and Germany suggest an equal or broader adoption of ocrelizumab as a first-line treatment in treatment-naïve patients compared to France (82.7% of naïve patients in Italy and 39.1% in Germany). 12-14 These differences might reflect not only divergent clinical guidelines but also distinct reimbursement frameworks and healthcare policies.15
Since our study is based on the national French healthcare claims database, we can reasonably assume that it includes nearly all MS patients in the country. While it includes some medical data, such as hospital diagnoses, it lacks most clinical information (especially expanded disability status scale) and also laboratory test results and imaging findings. As a result, data on disease activity and severity were not available. Furthermore, our study was purely descriptive, and no comparative analyses were conducted.
In conclusion, this study highlights the evolving landscape of MS treatment in France, emphasizing the predominant use of ocrelizumab and other high-efficacy DMTs among non-naïve patients facing active disease. Additionally, the high prevalence of psychiatric and neurological comorbidities among MS patients illustrates the need for comprehensive care strategies that address both physical and mental health. Future research should aim to explore the long-term impacts of different treatment regimens and the role of comorbidities, ultimately guiding more personalized approaches to MS management that enhance patient outcomes and quality of life.
Supplementary Material
Supplementary Table 1.
Treatments of interest in multiple sclerosis
Notes
Acknowledgements
The authors thank the French NHS (Caisse Nationale de l’Assurance Maladie) for providing access to its claims data.
Author Contributions
XM, GM, and SL supervised the study and contributed to results interpretation. MB contributed to study design and interpretation of data. FD performed data analysis. AC, DP, LR, and GB supervised the study, contributed to study design and results interpretation. CM and MB contributed to the study design and wrote the manuscript with input from all authors. All authors read and approved the final version of the manuscript.
Conflicts of Interest
MB, FD, and CM are full time employees of PELyon. AC, DP, LR, and GB are full time employees of Roche SAS. GM and SL have no conflicts of interest to declare. XM has received financial support from Allergan-Abbvie, Aptis Pharma, Biogen, BMS, Grünenthal, Lilly, Lundbeck, Teva, Merck-Serono, Novartis, Orion, Pfizer, Roche, and Sanofi-Genzyme and non-financial support from SOS Oxygène not related to the submitted work.
Funding Statement
This study was founded by Roche SAS.
Data Availability Statement
The datasets presented in this article are not readily available because, due to NHS and SNDS rules, no data sharing is possible as access to data is restricted to habilitated and qualified researchers (Floriane Deygas is habilitated and qualified).
Ethical Approval
This study was approved by the French Health Data Hub (approval no. 3471595bis from May 6, 2021) and the French Data Protection Authority (CNIL; approval no. 921221 from September 15, 2021).
Patient Consent for Publication
NA.