| J Mult Scler Neuroimmunol > Volume 16(1); 2025 > Article |
|
ABSTRACT
Background
Methods
Results
Supplementary Material
Supplementary Table 1.
NOTES
Acknowledgements
The authors thank the French NHS (Caisse Nationale de lŌĆÖAssurance Maladie) for providing access to its claims data.
Author Contributions
XM, GM, and SL supervised the study and contributed to results interpretation. MB contributed to study design and interpretation of data. FD performed data analysis. AC, DP, LR, and GB supervised the study, contributed to study design and results interpretation. CM and MB contributed to the study design and wrote the manuscript with input from all authors. All authors read and approved the final version of the manuscript.
Conflicts of Interest
MB, FD, and CM are full time employees of PELyon. AC, DP, LR, and GB are full time employees of Roche SAS. GM and SL have no conflicts of interest to declare. XM has received financial support from Allergan-Abbvie, Aptis Pharma, Biogen, BMS, Gr├╝nenthal, Lilly, Lundbeck, Teva, Merck-Serono, Novartis, Orion, Pfizer, Roche, and Sanofi-Genzyme and non-financial support from SOS Oxyg├©ne not related to the submitted work.
Data Availability Statement
The datasets presented in this article are not readily available because, due to NHS and SNDS rules, no data sharing is possible as access to data is restricted to habilitated and qualified researchers (Floriane Deygas is habilitated and qualified).
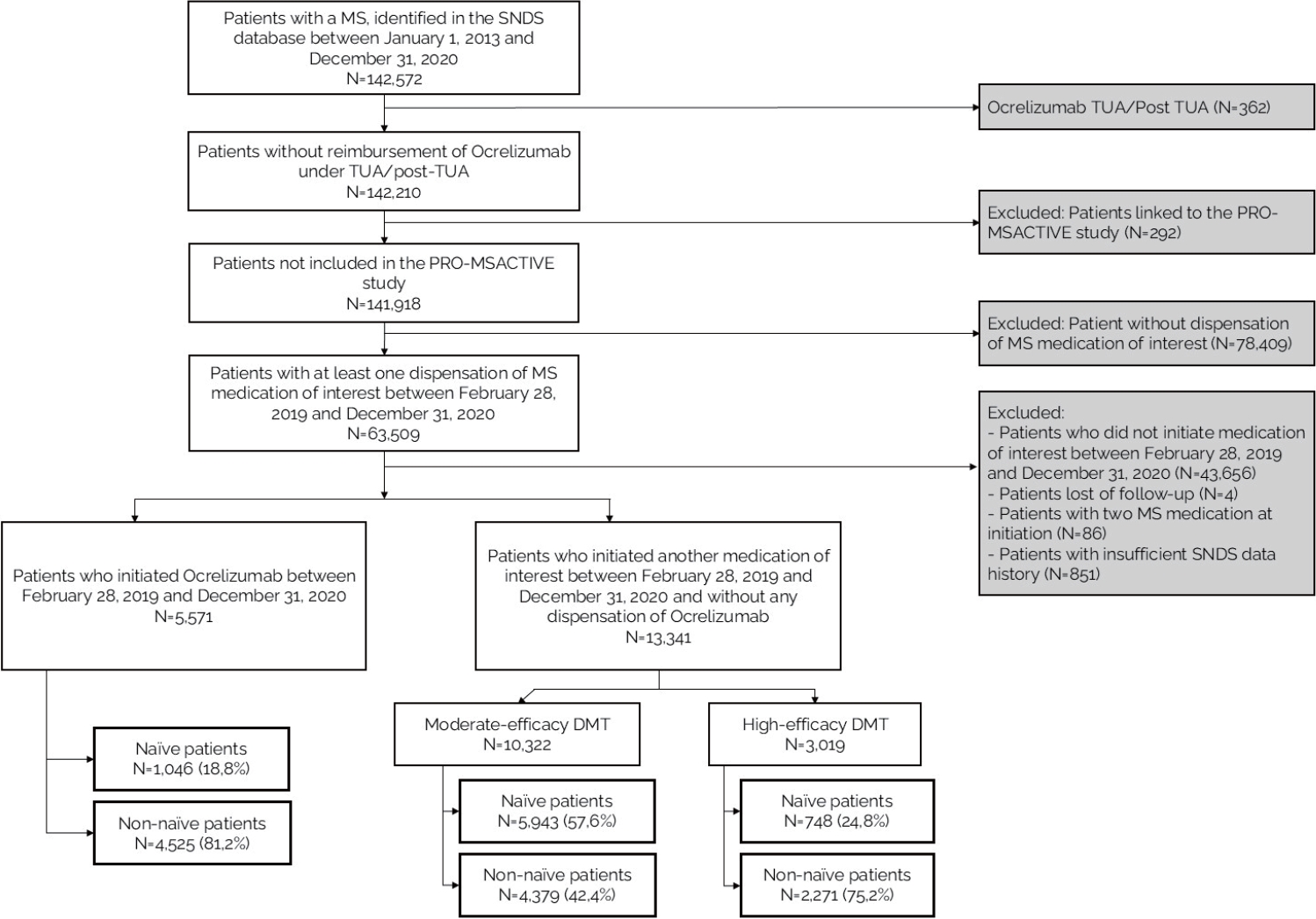
Figure┬Ā1.
Table┬Ā1.
| Overall (n=18,912) | Naive ocrelizumab (n=1,046) | Non-naive ocrelizumab (n=4,525) | Naive MEa DMT (n=5,943) | Non-naive MEa DMT (n=4,379) | Naive HEb DMT (n=748) | Non-naive HEb DMT (n=2,271) | |
|---|---|---|---|---|---|---|---|
| Female | 14,154 (74.8) | 678 (64.8) | 3,247 (71.8) | 4,478 (75.3) | 3,528 (80.6) | 515 (68.9) | 1,708 (75.2) |
| Mean age at index date | 41.3┬▒11.7 | 43.4┬▒13.4 | 42.8┬▒11.0 | 39.5┬▒11.9 | 43.4┬▒11.5 | 35.3┬▒12.6 | 40.1┬▒10.7 |
| Age at index date | |||||||
| ŌĆā<20 years | 432 (2.3) | 25 (2.4) | 36 (0.8) | 210 (3.5) | 19 (0.4) | 90 (12.1) | 52 (2.3) |
| ŌĆā20-39 years | 8,550 (45.2) | 403 (38.5) | 1,818 (40.2) | 2,975 (50.1) | 1,841 (42.0) | 403 (53.9) | 1,110 (48.9) |
| ŌĆā40-59 years | 8,633 (45.6) | 480 (45.9) | 2,360 (52.2) | 2,448 (41.2) | 2,099 (47.9) | 224 (29.9) | 1,022 (45.0) |
| ŌĆāŌēź60 years | 1,297 (6.9) | 138 (13.2) | 311 (6.8) | 310 (5.2) | 420 (9.7) | 31 (4.1) | 87 (3.8) |
| Free access to care status | 1,645 (8.7) | 122 (11.7) | 386 (8.5) | 537 (9.0) | 335 (7.7) | 87 (11.6) | 178 (7.8) |
| LTD status | 16,805 (88.9) | 900 (86.0) | 4,288 (94.8) | 4,691 (78.9) | 4,182 (95.5) | 593 (79.3) | 2,151 (94.7) |
| Time since the start of the LTD status and index date (in months) | |||||||
| ŌĆāMedian (Q1-Q3) | 55.5 (7.2-130.8) | 8.0 (3.0-105.3) | 104.9 (53.9-170.8) | 3.4 (1.5-10.7) | 89.7 (44.9-153.1) | 3.7 (2.0-8.7) | 73.7 (40.2-128.8) |
| Comorbidities | |||||||
| ŌĆāDiabetes (type 1 and 2) | 525 (2.8) | 40 (3.9) | 134 (3.0) | 142 (2.4) | 143 (3.3) | 20 (2.7) | 46 (2.0) |
| ŌĆāChronic diseases of the liver or pancreas and cirrhosis | 169 (0.9) | 15 (1.5) | 58 (1.3) | 38 (0.6) | 33 (0.8) | 7 (1.0) | 18 (0.8) |
| ŌĆāCardio-neurovascular | 1,076 (5.8) | 87 (8.4) | 286 (6.4) | 307 (5.2) | 280 (6.5) | 28 (3.8) | 88 (3.9) |
| ŌĆāPsychiatric/neurologic | 4,043 (21.6) | 253 (24.5) | 1,345 (30.0) | 873 (14.9) | 1,007 (23.2) | 101 (13.9) | 464 (20.6) |
| ŌĆāŌĆāDepression | 2,901 (15.5) | 169 (16.4) | 997 (22.2) | 581 (9.9) | 750 (17.3) | 68 (9.3) | 336 (14.9) |
| ŌĆāŌĆāAnxiety | 1,766 (9.5) | 112 (10.8) | 570 (12.7) | 419 (7.2) | 420 (9.7) | 48 (6.6) | 197 (8.8) |
| ŌĆāŌĆāBipolar disorder | 116 (0.6) | 8 (0.8) | 27 (0.6) | 34 (0.6) | 32 (0.7) | 7 (1.0) | 8 (0.4) |
| ŌĆāŌĆāEpilepsy | 197 (1.1) | 18 (1.7) | 61 (1.4) | 56 (1.0) | 41 (0.9) | 3 (0.4) | 18 (0.8) |
| ŌĆāŌĆāRestless leg syndrome | 74 (0.4) | 4 (0.4) | 30 (0.7) | 10 (0.2) | 19 (0.4) | 1 (0.1) | 10 (0.4) |
| ŌĆāAutoimmune disease | 252 (1.3) | 22 (2.1) | 62 (1.4) | 70 (1.2) | 52 (1.2) | 19 (2.6) | 27 (1.2) |
| ŌĆāSevere chronic respiratory insufficiency | 784 (4.2) | 43 (4.2) | 206 (4.6) | 247 (4.2) | 177 (4.1) | 31 (4.3) | 80 (3.6) |
| ŌĆāCancer | 483 (2.6) | 29 (2.8) | 118 (2.6) | 130 (2.2) | 139 (3.2) | 20 (2.7) | 47 (2.1) |
| ŌĆāMissing data | 225 (1.2) | 13 (1.2) | 38 (0.8) | 86 (1.4) | 46 (1.1) | 19 (2.5) | 23 (1.0) |
| Charlson comorbidity index (adjusted on age) | |||||||
| ŌĆā0 | 12,264 (64.8) | 511 (48.9) | 2,736 (60.5) | 4,086 (68.8) | 2,810 (64.2) | 491 (65.6) | 1,630 (71.8) |
| ŌĆā1-2 | 5,500 (29.1) | 381 (36.4) | 1,485 (32.8) | 1,555 (26.1) | 1,318 (30.1) | 192 (25.7) | 569 (25.0) |
| ŌĆā3-4 | 1,022 (5.4) | 134 (12.8) | 281 (6.2) | 268 (4.5) | 216 (4.9) | 56 (7.5) | 67 (3.0) |
| ŌĆāŌēź5 | 126 (0.7) | 20 (1.9) | 23 (0.5) | 34 (0.6) | 35 (0.8) | 9 (1.2) | 5 (0.2) |
| Charlson comorbidity index (unadjusted) | |||||||
| ŌĆā0 | 15,931 (84.2) | 740 (70.7) | 3,704 (81.8) | 5,036 (84.7) | 3,887 (88.7) | 550 (73.5) | 2,014 (88.7) |
| ŌĆā1-2 | 2,682 (14.2) | 265 (25.4) | 754 (16.7) | 818 (13.8) | 436 (10.0) | 166 (22.2) | 243 (10.7) |
| ŌĆā3-4 | 257 (1.4) | 38 (3.6) | 58 (1.3) | 79 (1.3) | 42 (1.0) | 26 (3.5) | 14 (0.6) |
| ŌĆāŌēź5 | 42 (0.2) | 3 (0.3) | 9 (0.2) | 10 (0.2) | 14 (0.3) | 6 (0.8) | 0 (0.0) |
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print